Bao-Liang Song
――――――――――――――――――――――――――――――――――――
Professor of Biochemistry
Dean of the College of Life Sciences
Affiliation: Dept.of Biochemistry
E-mail: blsong@whu.edu.cn
--------------------------------------------------------------------------------
Research Interests:
Cholesterol is an essential component of biological membranes, and is a precursor for synthesis of steroid hormones and bile acids. High level of cholesterol causes severe problems including coronary heart disease and stroke. Humans obtain cholesterol through de novo cholesterol biosynthesis and intestinal absorption. Understanding the regulatory mechanisms and molecular pathways of cholesterol metabolism will provide necessary basis for controlling metabolic diseases. Ongoing projects in this laboratory include:
1) Dissecting the molecular pathway of dietary cholesterol absorption.
2) Studying mechanism and function of intracellular cholesterol trafficking.
3) Uncovering the regulatory mechanisms of cholesterol metabolism.
4) Screening for small chemical compounds controlling cholesterol metabolism.
The long-term goals of these studies are to reveal the molecular mechanism of cholesterol metabolism and develop novel strategies to treat cholesterol-related diseases.
Education Background & Academic Experience:
Bao-Liang Song received a B.A. degree in Biology in 1997 from Nanjing University, China. He then entered a doctoral program at Shanghai Institute of Biochemistry and Cell Biology, China. He obtained his Ph.D. degree in 2002. From 2002 to 2005, Dr. Bao-Liang Song received his post-doctoral training in the laboratory of Drs. Joseph L. Goldstein and Michael S. Brown at UT Southwestern Medical Center, and worked with Dr. Russell DeBose-Boyd on the degradation of HMG-CoA reductase. He joined Shanghai Institute of Biochemistry and Cell Biology as Principle Investigator in 2005. He was appointed as Chief Scientist by the Ministry of Science and Technology of China in 2008, and won China National Funds for Distinguished Young Scientists in 2009. He won Tan KahKee Young Scientist Award in Life Sciences in 2012 and Arthur Kornberg Memorial Award in 2013.
Research Description:
Cholesterol is an essential component of mammalian membranes. But high level of circulating cholesterol causes cardiovascular disease. Imbalanced cholesterol metabolism is related to Alzheimer’s disease, cancer and other diseases. Aiming to address fundamental questions in the lipid field and improve human health, we focus on cholesterol metabolism and have made scientific achievements as follows.
1. Regulation of cholesterol biosynthesis and intestinal absorption.
(A) We identify a rare frameshift variant in the LIMA1 gene from a Chinese Kazakh family with inherited low plasma cholesterol. The loss-of-function mutation of LIMA1 reduces intestinal cholesterol absorption and lower plasma cholesterol, suggesting targeting LIMA1 can be a new way to lower cholesterol level (Science, 2018). (B) We have elucidated the molecular mechanism of intestinal cholesterol absorption. We find that NPC1L1 and Flotillins form cholesterol-rich microdomains in the plasma membrane (PNAS, 2011). When the cholesterol level is high, cholesterol binds to the N-terminal domain of NPC1L1 (JBC, 2011), releasing its C-terminal cytoplasmic tail to recruit the clathrin adaptor Numb and initiating clathrin-mediated endocytosis (Nat Med, 2014). The cholesterol absorption inhibitor ezetimibe blocks cholesterol uptake by impairing NPC1L1 endocytosis (Cell Metab, 2008). Cholesterol and fatty acids stabilize ACAT-2 that increases cholesterol absorption efficiency and prevents lipotoxicity (NCB, 2017). (C) The sterol-regulated degradation of HMG-CoA reductase (HMGCR) and the SREBP pathway are two major feedback regulatory mechanisms governing cholesterol synthesis. Together with others, we have delineated the HMGCR degradation pathway. We identify gp78 and RNF145 as the key E3s catalyzing HMGCR ubiquitination (Mol Cell, 2005; Cell Metab, 2012; JBC, 2018), characterized lanosterol, 24,25-dihydrolanosterol and geranylgeraniol as the endogenous regulators (JBC, 2003; Cell Metab, 2005), reveal that Ufd1 is a cofactor of gp78 (Cell Metab, 2007). (D) Based on these findings, we have developed compounds to treat hyperlipidemia by inhibiting SREBP processing or inducing HMGCR degradation (Cell Metab, 2011; Nat Commun, 2018).
2. Cholesterol transport through lysosome-peroxisome membrane contacts.
Most mammalian cells take up cholesterol from low-density lipoprotein (LDL) through receptor-mediated endocytosis. After reaching lysosomes, LDL-derived cholesterol continues to move to other organelles. However, to where and how cholesterol moves from lysosome are poorly understood. We find that cholesterol can be conveyed through membrane contact sites formed between lysosomal protein synaptotagmin VII (Syt7) and peroxisomal lipid PI(4,5)P2 (Cell, 2015). We further identify that the GARP complex is involved in cholesterol transport by targeting NPC2 to lysosomes (Cell Rep, 2017). We have reviewed the cholesterol transport at membrane contact sites (TiBS, 2018).
3. Covalent cholesterol modification of Smoothened (SMO).
Hedgehog (Hh) has been known as the only cholesterol-modified morphogen playing pivotal roles in development and tumorigenesis. A major unsolved question is how Hh signaling regulates the activity of SMO. Through an unbiased biochemical screen, we identify that SMO is covalently modified by cholesterol on the Asp95 (D95) residue through an ester bond. This modification is inhibited by Patched-1 (Ptch1) but enhanced by Hh. The cholesterylation of SMO is critical for Hh signal transduction, assuring proper embryonic development (Mol Cell, 2017). This work reveals a novel function of cholesterol as a covalent ligand of SMO to regulate cell fate.
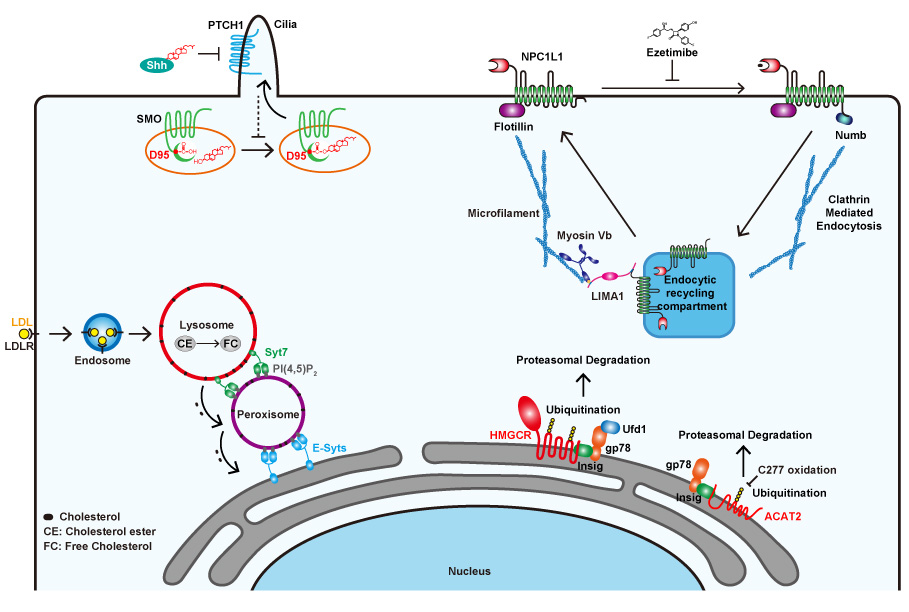
Figure 1. Summary of Song’s work on cholesterol metabolism.
Representative Publications (#: Co-first; *: Co-corresponding):
1) Luo J, Yang H and Song BL*. Mechanisms and regulation of cholesterol homeostasis. Nature Reviews Molecular Cell Biology. 21(4): 225-245, 2020
2) Xiao J#, Luo J#, Hu A, Xiao T, Li M, Kong Z, Jiang L, Zhou Z, Liao Y, Xie C, Chu B, Miao H, Li B, Shi X and Song BL*. Cholesterol transport through the peroxisome-ER membrane contacts tethered by PI(4,5)P2 and extended synaptotagmins. Sci China Life Sci., 62(9):1117-1135, 2019
3) Gong XM#, Li YF#, Luo J#, Wang JQ#, Wei J#, Wang JQ, Xiao T, Xie C, Hong J, Ning G, Shi XJ, Li BL, Qi W* and Song BL*. Gpnmb secreted from liver promotes lipogenesis in white adipose tissue and aggravates obesity and insulin resistance. Nature Metabolism, 1: 570-583, 2019
4) Zhang YY#, Fu ZY#, Wei J#, Qi W, Baituola G, Luo J, Meng YJ, Guo SY, Yin H, Jiang SY, Li YF, Miao HH, Liu Y, Wang Y, Li BL, Ma YT* and Song BL*. A LIMA1 variant promotes low plasma LDL cholesterol and decreases intestinal cholesterol absorption. Science, 360(6393):1087-1092, 2018
5) Wang YJ, Bian Y, Luo J, Lu M, Xiong Y, Guo SY, Yin HY, Lin X, Li Q, Chang CCY, Chang TY, Li BL* and Song BL*. Cholesterol and fatty acids regulate cysteine ubiquitination of ACAT2 through competitive oxidation. Nature Cell Biology, 19(7): 808-819, 2017
6) Xiao X#, Tang JJ#, Peng C, Wang Y, Fu L, Qiu ZP, Xiong Y, Yang LF, Cui HW, He XL, Yin L, Qi W, Wong CL, Zhao Y, Li BL, Qiu WW* and Song BL*. Cholesterol modification of Smoothened is required for hedgehog signaling. Molecular Cell, 66: 154-162, 2017
7) Chu BB#, Liao YC#, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL and Song BL*. Cholesterol Transport through Lysosome-Peroxisome Membrane Contacts. Cell, 161(2): 291-306, 2015
8) Li PS, Fu ZY, Zhang YY, Xu CQ, Ma YT, Li BL and Song BL*. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nature Medicine, 20(1): 80-86, 2014
9) Liu TF, Tang JJ, Li PS, Shen Y, Li JG, Miao HH, Li BL* and Song BL*. Ablation of gp78 in liver improves hyperlipidemia and insulin resistance by inhibiting SREBP to decrease lipid biosynthesis. Cell Metabolism, 16: 213-225, 2012
10) Tang JJ#, Li JG#, Qi W, Qiu WW, Li PS, Li BL and Song BL*. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metabolism, 13: 44-56, 2011
11) Ge L#, Wang J#, Qi W#, Miao HH, Cao J, Qu YX, Li BL and Song BL*. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metabolism,7: 508-519, 2008
12) Cao J, Wang J, Qi W, Miao HH, DeBose-Boyd RA, Wang J, Li BL* and Song BL*. Ufd1 is a cofactor of gp78 and plays a key role in cholesterol metabolism. Cell Metabolism, 6:115-128, 2007
13) Ge L, Qi W, Wang LJ, Miao HH, Qu YX, Li BL and Song BL*. Flotillins play an essential role in Niemann-Pick C1 Like 1-mediated cholesterol uptake. PNAS, 108(2): 551-6, 2011
14) Song BL, Sever N, and DeBose-Boyd RA*. Gp78, a membrane anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Molecular Cell. 19(6):829-840, 2005
15) Song BL, Javitt NB, and DeBose-Boyd RA*. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metabolism, 1: 179-189, 2005
16) Sever N#, Song BL#, Yabe D#, Goldstein JL*, Brown MS*, and DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of mammalian 3-Hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J Biol Chem, 278: 52479-52490, 2003

